The ‘improved’ sunscreen labeling system introduced in 2011 by the FDA may not be as effective as intended. A study has shown that consumers do not fully understand the most important pieces of information, such as SPF, on the packaging.
Some of the new rules for sunscreen labels included:
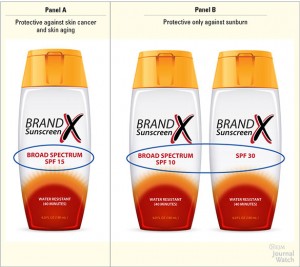
- The term “broad spectrum” may only appear on sunscreens proven to protect against both UVA and UVB rays.
- “Waterproof”, “sweatproof” and “sunblock” can no longer be used on the label, as these words tend to overstate the product’s effectiveness.
- Only broad spectrum sunscreens with an SPF of 15 or higher can claim to reduce the risk of skin cancer and early skin aging if used as directed.
- Sunscreen cannot claim to provide protection for more than two hours without reapplication, or claim to provide “instant” protection without first submitting evidence to the FDA.
In a recent article in NEJM Journal Watch (July 16, 2015), reports that the 2014 study, published in June 2015, identifies that this long-overdue labeling change has fallen short.
The key issues discovered in the study:
- Many people don’t understand the difference between UVA and UVB, and the importance of using a “broad spectrum” (which includes UVA protection) sunscreen.
- Only a small percentage (38%) knew that high SPF or broad spectrum sunscreens protect against skin cancer.
- Choice of sunscreen tends to be driven by high SPF, sensitive-skin formulation and water/sweat resistance features.
The article suggests that a publicity campaign is needed to properly educate consumers on how read sunscreen labels properly and select the “most suitable sunscreen.”